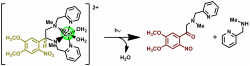Overview
Research in the Burdette group involves a multidisciplinary approach to studying problems at the interface of metals, biology and photochemistry. Most projects begin with synthesis of chemical tools that are designed to execute a particular task such as bind and release a metal ion of interest. The new chemical tools are then characterized spectroscopically and applied to answering a question in a related field like biology either within the group or in collaboration with outside experts.
Photocaged Complexes
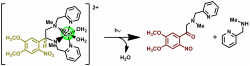
|
Photocaged Complexes are metal ion chelators that undergo photochemical reactions upon exposure to light. The light-induced structure changes weaken the ligand-metal interaction, which shifts the binding equilibrium toward free (solvated) metal ions. The Burdette group has developed Cast, Cleav and deCage photocages for Zn2+, Cu+ and other biologically relevant metal ions. Photocaged complexes can be used to study metal ion homeostasis and metal-based neurotransmittion.
|
|
Azobenzene Photochemsitry
|
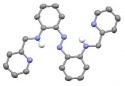
Azobenzene photochemsitry is one of the most important subjects in photochromism research. Azobenzene consists of two pheny rings conected by a diazene group. While the trans isomer is the most stable, exposure to results isomerization to give the cis isomer. Substitution on the aromatic rings modulates the photoisomerization of azobenzene derivatives. The Burdette group has studied a variety of aminoazobenzene derivatives that exhibit unexpected photochemistry and are interested in exploiting this behavior for different applications.
|
|
Photoactive MOFs
The Periodic Table
Polymeric and Small Molecule Fluorescent Sensors
| The Burdette group has experience with both polymeric sensors and small molecule sensors. The emphasis of these projects is to develop approaches to imaging metal ions that usually quench fluorescence emission. Polymeric sensors are being developed for environmental monitoring of effluent waters since excess Cu(II) is toxic to aquatic life. Small molecule sensors are being developed for imaging iron in cells and biological tissues. Iron may be involved in the pathology of several neurodegenerative disorders like Alzheimer's disease through uncontrolled free radical formation by Fenton chemistry. Both copper and iron quench the fluorescence of traditional sensors. This research program is currently idle.
|
|