Batteries
A battery is a device consisting of electro-chemical cells that convert stored chemical energy into electrical energy. There are numerous types of batteries, all with there own pro's and cons.Primary batteries get there power from the chemicals added during assembly and cannot be recharged. Secondary batteries require a charge after assembly before they can output electricity. We will primarily cover secondary batteries on this page.
Contents
Lead acid battery
Moderately expensive. Moderate energy density. Moderate rate of self-discharge. Higher discharge rates result in considerable loss of capacity. Environmental hazard due to Lead. Common uses include automobile batteries, motorcycle, and ATV batteries.
NiCad
Inexpensive. High-/low-drain, moderate energy density. Can withstand very high discharge rates with virtually no loss of capacity. Moderate rate of self-discharge. Environmental hazard due to Cadmium – use now virtually prohibited in Europe
NiMh
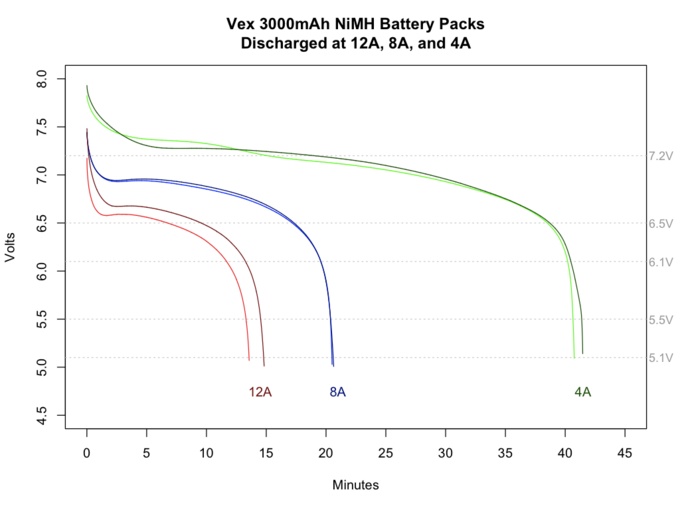
Nickel metal hydride batteries are used in the RBE kit. The 7.2V batteries will actually read 7.2V with no load when they are depleted. The discharge graph below is for a perfectly charged, new battery in ideal situations.
Inexpensive. Performs better than alkaline batteries in higher drain devices. Traditional chemistry has high energy density, but also a high rate of self-discharge. Newer chemistry has low self-discharge rate, but also a ~25% lower energy density. Used in some cars.
Lithium Ion
Very expensive. Very high energy density. Not usually available in "common" battery sizes. Very common in laptop computers, moderate to high-end digital cameras, camcorders, and cellphones. Very low rate of self-discharge. Terminal voltage unstable. Volatile: Chance of explosion or self fueling fire if short-circuited, allowed to overheat, are physically damaged, or not manufactured with rigorous quality standards.